| . |  |
. |
Washington (AFP) Dec 23, 2009 A week after 800,000 doses of children's swine flu vaccine were recalled in the United States for losing their potency, a second vaccine maker recalled its A(H1N1) nasal spray for the same reason. MedImmune announced late Tuesday that it was "voluntarily recalling unused doses of 13 specific lots of influenza A(H1N1) 2009 Monovalent Vaccine Live, Intranasal due to a slight decrease in potency. "There is no safety concern with the lots that are being recalled," it added in a statement. The recall involves doses with best-before dates between January 19 and January 26, 2010. MedImmune's nasal spray vaccine was the first to become available in the United States, but can only be administered to children older than two and healthy adults up to age 50. According to Food and Drug Administration spokeswoman Karen Riley, the recall involves 3,000 doses of the nasal spray still at a central distribution center and an unknown number at healthcare facilities or with health care authorities in various states. "Given the intense demand in October and November, we don't expect there is much left with healthcare facilities or in the states," said Riley. Children and adults who had the nasal spray vaccine in the first months of the vaccination campaign in the United States do not need to be vaccinated again, MedImmune said. Last week, Sanofi Pasteur recalled 800,000 doses of injectable swine flu vaccine for children after routine tests showed they had lost potency. Like the MedImmune vaccine that was recalled Tuesday, Sanofi Pasteur said its treatment lost minimal strength and that children already inoculated with vaccine from the recalled lots would not require a second round of vaccinations.
New antiviral effective against bird flu T-705 even works after several days of infection, said Yoshihiro Kawaoka, a virologist at the University of Wisconsin's School of Veterinary Medicine. "H5N1 virus is so pathogenic even Tamiflu doesn't protect all the infected animals," Kawaoka said. "This (antiviral) compound works much better, even three days after infection." T-705 has been tested successfully against bird flu and H1N1 swine flu in mouse experiments and is being tested on humans in Japan against seasonal flu, Kawaoka said in the Proceedings of the National Academy of Sciences. Some scientists believe bird flu, which is extremely difficult to treat, could spread worldwide, though so far cases have been isolated to Asia. T-705 targets the viral molecule polymerase, an enzyme that enables the virus to copy its genetic material. By disabling polymerase, the virus can't create new virus particles needed to maintain the chain of infection, Kawaoka said in a release Tuesday. Share This Article With Planet Earth
Related Links Epidemics on Earth - Bird Flu, HIV/AIDS, Ebola
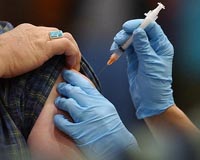 At least 60 million in US have had swine flu shot: CDC
At least 60 million in US have had swine flu shot: CDCWashington (AFP) Dec 22, 2009 At least 60 million people in the United States have been vaccinated against swine flu, and children are twice as likely as adults to have been innoculated, a top health official said Tuesday. "We think right now at least 60 million people have been vaccinated" against pandemic H1N1 flu, said Anne Schuchat from the US Centers for Disease Control and Prevention (CDC). Her findings were ... read more |
|
| The content herein, unless otherwise known to be public domain, are Copyright 1995-2009 - SpaceDaily. AFP and UPI Wire Stories are copyright Agence France-Presse and United Press International. ESA Portal Reports are copyright European Space Agency. All NASA sourced material is public domain. Additional copyrights may apply in whole or part to other bona fide parties. Advertising does not imply endorsement,agreement or approval of any opinions, statements or information provided by SpaceDaily on any Web page published or hosted by SpaceDaily. Privacy Statement |